Cansino Vaccine Europe
CanSino Biologics Inc. CanSino Biologics simplified Chinese.
Covid 19 Vaccines Made By China S Sinopharm Cansino Release Efficacy Data South China Morning Post
Chinas CanSino Biologics Inc CanSinoBIO 6185HK said on Monday its COVID-19 vaccine has been authorised for emergency use in Hungary the second Chinese vaccine to receive approval in that.

Cansino vaccine europe. Moroccan and Egyptian companies aim to start producing Chinas Sinopharm and Sinovac vaccines respectively while Rwanda sign ed a deal with the. So far these include the vaccines. EU countries officially agreed on Thursday to welcome foreign travelers who have received one of the coronavirus vaccines approved by European regulators.
COVID-19 vaccines reviewed for use in the EU under Article 5 3 of Regulation 7262004. CanSinos so-called viral vector vaccine which loads an antigen from the coronavirus on a harmless cold-causing pathogen called adenovirus was the first in the world to start human clinical. Randomisation and masking The Ad5-vectored COVID-19 vaccine was developed by the Beijing Institute of Biotechnology Beijing China and CanSino Biologics and contained replication-defective Ad5 vectors expressing the full-length spike gene based on Wuhan-Hu-1 GenBank accession number YP_009724390.
Is monitoring its Covid-19 vaccine more carefully after cases of blood clots forced other suppliers to suspend inoculations. La technologie de vaccin développée par Cansino repose sur lutilisation dun vecteur viral. Detailed information on the development evaluation approval and monitoring of COVID-19 vaccines in the EU.
Trade-named Convidecia is a single-dose viral vector COVID-19 vaccine developed by CanSino. The JJ vaccine has been cleared for use in countries across Europe the US. The EU GMP certification is required to import COVID-19 vaccines into the European Union and is regarded as a recognition of leading industry standards by many authorities outside of the EU.
Key facts about COVID-19 vaccines in the EU. The CanSino vaccine has already been granted approval in China and is authorized for emergency use in Hungary Pakistan and Mexico. Hungary has granted emergency use authorization for the inoculation making CanSino the second China-developed COVID-19 vaccine approved in the European country said Hungarian Chief Medical Officer Cecilia Muller on Monday.
The CanSino vaccine has already been granted approval in China and is authorized for emergency use. Detailed information on the studies EMA requires to confirm a vaccine is safe provides adequate. Since late 2020 it has been in Phase III trials in Chile Mexico Pakistan Russia and Saudi Arabia with 40000 participants.
The CanSino vaccine has already been granted approval in China and is authorized for emergency use in Hungary Pakistan and Mexico. Were just monitoring more cautiously CanSino. EMA is not involved in advising on travel requirements in the EU such as vaccination quarantine or testing for travellers.
In August 2020 CanSinoBIO entered into a strategic partnership with Shanghai Pharmaceuticals Chinas second largest pharmaceutical company in fast expanding production capacity storage and. The JJ vaccine has been cleared for use in countries across Europe the US. The company is seeking Emergency Use Listing with the World Health Organization which would pave the way for the shot to be distributed through the.
They work by taking a virus known to cause common colds and. The products made by CanSino AstraZeneca Johnson and Johnson and Russias Gamaleya Institute are all adenovirus-vectored vaccines. Un virus modifié autre que le coronavirus SARS-CoV-2 rendu incapable de se répliquer et porteur.
The Canadian government allows eligible travelers who have received the full series of an accepted COVID-19 vaccine or a combination of accepted vaccines. Vaccines authorised in the European Union EU to prevent COVID-19 following evaluation by the European Medicines Agency EMA. AD5-nCOV trade-named Convidecia is a single-dose viral vector vaccine for COVID-19 developed by CanSino Biologics.
The vaccine called Ad5-nCoV or Convidecia which the company CanSino Biologics developed together with the Beijing Institute of Biotechnology is a vector virus vaccine based on an adenovirus type. In February 2021 global data from Phase III trials and 101 COVID cases showed that the vaccine. It conducted its Phase III trials in Argentina Chile Mexico Pakistan Russia and Saudi Arabia with 40000 participants.
The JJ vaccine has been cleared for use in countries across Europe the US. In February 2021 global data from Phase III trials and 101 COVID cases showed that the vaccine had a 657 efficacy in preventing.
Shepherding White Hot Global Vaccine Battle China Injects New Energy In The World S Fight Against Covid 19 Observers Global Times
/cloudfront-us-east-2.images.arcpublishing.com/reuters/3JK5OJZMV5N5TCCL7XMBR4MBYU.jpg)
Pakistan China To Gift Half Million Doses Of Sinopharm Vaccine Reuters

How Close Is A Coronavirus Vaccine Free To Read Financial Times

42 Cansinobio Files Application For Covid 19 Vaccine Approval As Trials Show 65 Efficacy Rate

China S Cansino Covid 19 Vaccine Wins Emergency Approval In Mexico South China Morning Post
Mexico Agrees To Buy 35 Million Doses Of Cansino Covid Vaccine World News Hindustan Times

Covid 19 Vaccine Candidates Idea Initiative

Cansinobio Covid 19 Says Booster Shot Can Reverse Drop In Antibodies Reuters
Vaccine Checker Which Vaccine Is Approved For International Travel
Pakistan To Import Chinese Cansino Covid Vaccine In Bulk To Package 3 Million Doses Locally Minister Reuters
Latest Biden Administration Expects To Begin Offering Booster Shots In September

China S Cansino Says European Vaccine Order Talks Are Underway Caixin Global
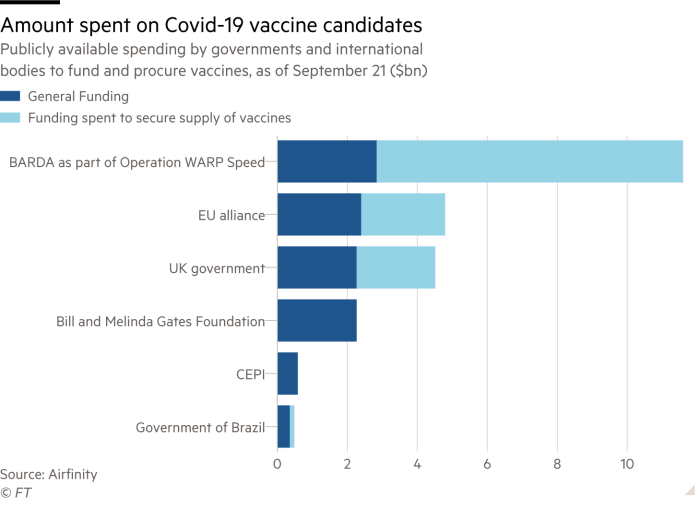
How Close Is A Coronavirus Vaccine Free To Read Financial Times

Coronavirus How Effective Are The Chinese Vaccines Science In Depth Reporting On Science And Technology Dw 01 02 2021
Cansino Granted Gmp Certificate From Hungary After Sinopharm Increasing Eu Confidence In Recombinant Covid 19 Vaccine Global Times
Covid 19 Vaccines In Mexico Should I Get Vaccinated Is It Safe Tecnologico De Monterrey
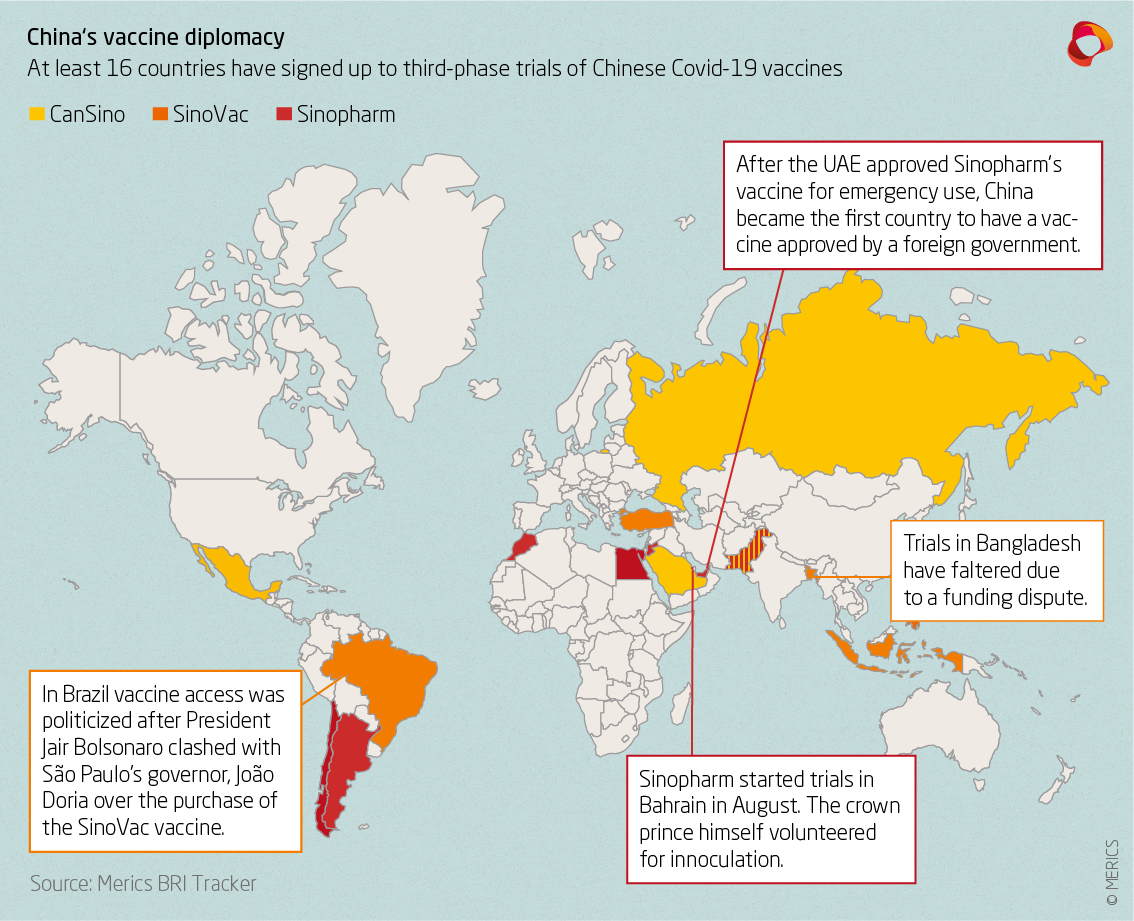
China S Vaccine Diplomacy Assumes Geopolitical Importance Merics

Everything You Need To Know About China S Coronavirus Vaccines Politico
Chinese Health Experts Advise Australia To Halt Approval For Pfizer Vaccine Following Norwegian Deaths Global Times
0 Response to "Cansino Vaccine Europe"
Posting Komentar